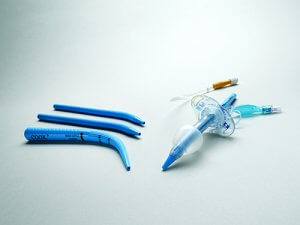
Limerick, Ireland — Cook Medical today announced that the Blue Rhino G2-Multi Percutaneous Tracheostomy Introducer sets and trays are commercially available to physicians in select markets in Europe and the Middle East. This product is a new iteration of the Ciaglia Blue Rhino product family and is designed to fit a wide range of tracheostomy tube sizes.
For more than 30 years, Cook has been a pioneer when it comes to innovating percutaneous dilational tracheostomy and airway management products. The predecessor product of Blue Rhino, a tracheostomy introducer set developed with Dr. Pasquale Ciaglia, included a series of dilators intended to be used sequentially to create an opening in the airway so that a tracheostomy tube could be inserted. Later, the introducer set was consolidated into a single product now known as the Blue Rhino. The grips and grooves on the product’s unique design were developed with Dr. Dietmar Enk. Today’s Blue Rhino G2-Multi introducer dilator is shaped with a gradual taper for single-stage dilation. The product comes in a variety of kit configurations to accomodate physicians’ preferences, enabling them to perform tracheostomies at the bedside in the ICU.
The new loading dilators in the Blue Rhino G2-Multi Percutaneous Tracheostomy Introducer sets and trays have been designed to fit with Medtronic’s newest line of tracheostomy tubes for most adult sizes: the Shiley™ Adult Flexible Tracheostomy Tube with TaperGuard™ Cuff, Disposable Inner Cannula and Shiley™ Adult Flexible Evac Tracheostomy Tube with TaperGuard™ Cuff and subglottic secretion management. Each set and tray comes with a range of loading dilators to help physicians find the right fit for the patient.
“Cook Medical has seen a significant increase in demand for our Blue Rhino products since the onset of the COVID-19 pandemic,” said Andy Cron, vice president for Cook’s Critical Care business. “Tracheostomies are performed on patients who need long-term ventilation, such as those suffering from acute respiratory failure. Percutaneous tracheostomies are associated with fewer bleeding complications.1 The Blue Rhino can be used at the patient’s bedside, eliminating the need to transport these critically ill patients to the operating room for a surgical procedure.”
“Even after 30 years of selling this product, we are still looking for ways to innovate and make our patients’ experiences better,” said Cron. “This is an example of Cook listening to patient and physician feedback and creating a new version that is compatible with more tracheostomy tubes.”
To learn more, visit CookMedical.eu/BlueRhinoMulti.
About Cook Medical
Since 1963, Cook Medical has worked closely with physicians to develop technologies that eliminate the need for open surgery. Today we are combining medical devices, biologic materials and cellular therapies to help the world’s healthcare systems deliver better outcomes more efficiently. We have always remained family owned so that we have the freedom to focus on what we care about: our patients, our employees, and our communities. Find out more at CookMedical.eu, and for the latest news, follow us on Twitter, Facebook and LinkedIn.
1 Delaney A, Bagshaw SM, Nalos M. Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: a systematic review and meta-analysis. Crit Care 2006;10(2):R55-R55.